|
|
|
|
We have already talked about light many times, but I have recieved so many questions about it that I will try to give a logical and understandable answer for our Rearers to these questions in this page. There will be even more pages about life too, but light, energy, is also a very important element of life, and so many questions need to be clarified about light.A lot of books have been written on light. But I did not find answers to these questions at all. They were only beating around the bush. The texts just deal with experiential facts, and theoretical questions
rarely give a meaningful answer. Light itself is something spatial,
and that is a very tricky thing. As I have already described, energy
is nothing more than a self-entangled time loop (which is quite a
wild thing) but it is itself the cause of itself. It feeds on tis own past.
This is true for all time loops, that is why physicists and
mathematicians working with relativity theory toss them. They
think it's a bad result. However, this is the key to a good and real
(and effective) solution. So the kid is thrown out of the window with
the bath water. Their prejudice is stronger than their
adventuresomeness. |
|
|
|
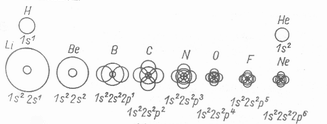
If no other kind of polarizer is available, I will just make one that will prove all this, and this is not a problem at all, since we get a different kind of suggestion from nature since the color and density of chrystals show a correlation which, as we know, depends on the atomic mass of atoms in them. It is interesting how they pull in "closer and closer" their electron shells as the number of nucleons increase. As a reminder, I will recall this from the previous page, because that is a very important thing. it gives clues about atoms. |
|
Light with very short wavelengths typically travels
through low specific weight materials. Yet milk is white and it hardly lets
light pass trough. Red flowers are red because they absorb the other colors
while red cellophane is red because it only allows the red color to pass.
Gamma radiation is only absorbed well by lead or uranium. Others do as well
but usually that is dangerous in itself. Therefore, lead is used. This is
cheaper and is not easily decomposed because it is the end point of almost
every atomic decomposition process. It will no longer decompose. It is a soft
metal which can be cut or carved with a knife. This amorphous softness also
contributes to its absorbing properties because its atomic disorder makes it
difficult for light to penetrate the tangled crystal lattice. Incredible as it
may be, but water absorbs a lot of the dangerous radiation, though it's a good
light transmitter in visible light. The window glass is also quite amorphous,
yet transparent. Annoying. So what makes any mater pass the light trough or
why does it absorb it? Some of the questions may seem completely amateurish,
since the scholars of light science have been able to answer these questions
for a hundred years. Really? |
|
|
|
|
|
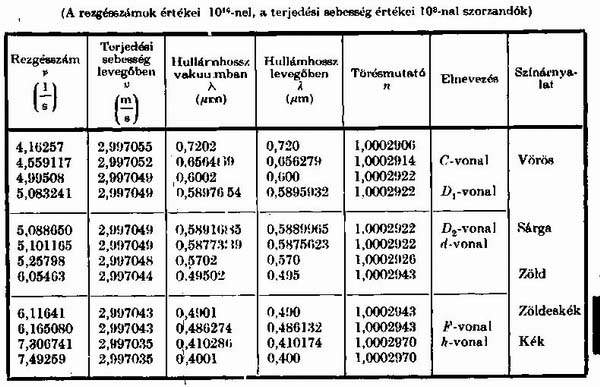
These data by themselves can point to many interesting things. Red light moves in the air at 299705,500 km/s and blue at 299703,500 km/s. Their speed difference is 2 km per second. Its refractive index is 1,0002906 and 1,0002970. Given that we see only a very small spectrum portion, this difference is also remarkable when we think of how wide the band of color spectrum invisible for us is. They claim that the velocity of different light quanta does not differ in vacuum. According to the table, their wavelength differs somewhat, which later on could turn out to be very important. |
|
|
|
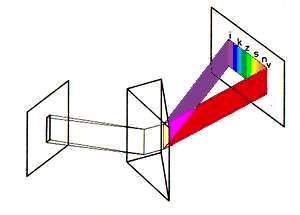
It can be seen very well in the textbook diagram that red (v) light from the
white light which
has been broken by the prism - which is faster even in air according to the
table - is bent les
by the prism than violet which is supposed to have higher energy (i). Well, my
question is,
which bullet is a bullet bullet with a bigger energy; the one that just drops
in front of my feet
or the one that reaches the target. Everyone will vote for the one that hits
the mark, right?
And how so with light? The faster and less bending one, or the light quantum
which is more
sluggis? If, however, the red one has more energy then why does the blue one
penetrate
deeper into the mater? Is it because the blue lightquantum is smaller than the
red one, and
therefore it can penetrate more effectively between the atoms and into the
nucleons
themselves? This seems more likely. |
|
The smaller light quantum more easiely penetrates under the scaly surface of the nucleons, but it is easier for it to leave it. After Chernobyl, an Ukrainian research director visited me (Dr. Gulyas). He asked me for advice as to how quickly could the soils be freed from radiation. I advised him to do a lot of (per week), sprinkling with dilute boric acid solution and pray for a lot of sunshine. At that time, radiation will first intensify, then to decrease rapidly. |
|
|
He
phoned me later that it worked. This was a double
treatment. Sunlight fills up the isotope atoms with light
at all wavelengths yet opens up their scales so that
Lasting long l but not infinitely. It's not an
electromagnetic wave. The color and wavelength of the
light result from the mechanical diameter of the time
loop. I do not know yet whether there are light quanta
created in a separate plane and ones created in a
separate space, or whether the one and the same kind
oscillates between the plane and the spatial vibration
states. The latter seems more likely. We are now
preparing to take a series of measurements to
determine this. We've got some raheel, down-to earth
tips. "Color" polarizers are expected to (partially) give
an answer to this question. |
|
|
If we look at the stars through the gas-medium of the universe (and we cannot do otherwise), the red light is most likely to come to our eyes from a star because the blue light quanta are scattered throughout their journey. The fact that we see them whitish is just because there is too little light four our light-seeing eyes (our light cones) and we only see "black and white". Photographs with long exposures will reveal a wonderful and colorful universe. That is, the stars scintellate in all colors. Yeah, that does not change that blue light is dispersed the most and red the least. I would like these astronomical colleagues and our cosmologists to think about it. This undeniable medium dispersion basically changes our image and perception of the universe around us. The M 20-21 dust galaxy, due to the large mass of illuminated material, dazzles in the sky with spectacular colors. |
|
|
If we look at the stars through the gas-medium of the universe (and we cannot do otherwise), the red light is most likely to come to our eyes from a star because the blue light quanta are scattered throughout their journey. The fact that we see them whitish is just because there is too little light four our light-seeing eyes (our light cones) and we only see "black and white". Photographs with long exposures will reveal a wonderful and colorful universe. That is, the stars scintellate in all colors. Yeah, that does not change that blue light is dispersed the most and red the least. I would like these astronomical colleagues and our cosmologists to think about it. This undeniable medium dispersion basically changes our image and perception of the universe around us. The M 20-21 dust galaxy, due to the large mass of illuminated material, dazzles in the sky with spectacular colors. If you like astronomy, check out our start page or go to Anita's astronomical websites |
|
|
Light is energy itself. It is not only informative through its color. Each light quanta brings us the message of its starry world, its planetary system, the living creatures there, and all their thoughts. We can not understand this with our instruments yet, but there is a mysterious something in our soul that may even interpret the beauty of the universe to its infinity and the thoughts of the created creatures in it. |
|
|
ALL RIGHTS RESERVED © 2007 UNIVERSUM UNIVERSITAS. This
publication was prepared for personal use only. |
|
 WORDTIMES 36.
WORDTIMES 36.