Wordtimes 43.
Wordtimes 43. ATOMS GETTING CLOSE AND GETTING AWAY FROM EACH OTHER |
|
|
We have already dealt with the question before, but there are many loose ends left dangling. Let's look at the phenomena one by ane and maybe we can incorporate the theoretical philosophy into practical life. Let's look at some practical examples for this! Those that are actually close to the problems of everyday life. |
|
|
Bendig.
|
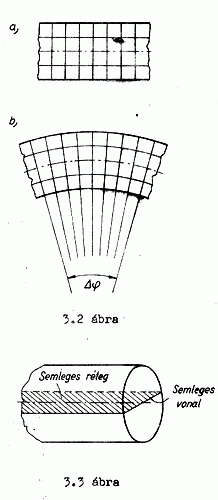
BENDING The evenly arranged atoms are located essentially at the same distance from each other in our theoretical model. I say theoretical because several effects are exerted even on a body at rest from the environment. Its temperature is not evenly the same since the side which gets more light will be the warmer one. If it is warmer then that side will also stretch. The atoms at the bottom are under more pressure than the ones at the top. This thing causes all kinds fo dilations which we cannot ignore. So, if we bend our body now, the
atoms on the convex side will get more separated from each other, and they
will clump togeother, approach each other on the concave side. It
obviously follows from this that where they get farther from oeach other (get
less dense) they will cool down there because it can absorb further light
quanta from the environment, whereas, wherever they get closer to each
other light will get pressed out of them. The light that leaves it will
carry the material's characteristics as well. The reason is that the light
emitted by the nucleons (nuclei) are modulated by the circulatory rhythm
and at the same time the cyclic dispersion of the electrons falling in
their path. That's how the spectra emerge. This can easily be verified
with an infrared spectral analysis. |
|
The light in the material which is also involved in the whole thing - since it is in an almost indispensable symbiosis with the nucleons - does not get pressed out of the the elastic materials to such an extent and thus keeps its elastic state for a long time because it can return to its original position. We can also see that a large part of the plastics age because they slowly lose the plasticizer materials and thereby (as they lose atoms and therefore they lose wave-generating systems in comparison to their initial state) their original bonds which were formed in a freshly manufactured state break down. For every material sublimates to some extent. That's why we smell their scent. This odor characteristic of the material is intensified during all kinds of material processing. Because when this happens the material loss is increased. |
|
|
ELONGATION |
|
|
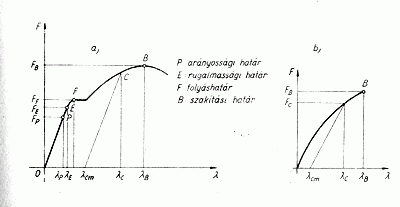
It can be seen well when stretching any material, though heavily dependent on the kind of material used, yet there is a linear phase in which the substance extends proportionally, then reaches the elastic limit at point E, and then shortly thereafter flows at point F and here it suddenly begins to disproportionately stretch. By then it has been irreversibly deformed. Here the material's atoms are torn away suddenly from each other, because the interferences surrounding each atom are by this time are no longer sufficient to keep the atoms of the material together. |
|
|
The principle can be freely applied
during all mechanical interventions. Rhythmic load exacerbates the
material much more intensely, than static loads because it speeds up the
rate at which the light keeps getting pushed out by many times. A good
example of this is the air humidifier ultrasonic equipment, creating a
vapor-like fume from the water. There is also a mechanical effect here
which tears the water droplets out of the water and it indeed leaves the
surface of water eventually. This is not sublimation in the traditional
sense and it is not steam eather. Water will leave the surface of the
water in the forom of liquid spray. |
|
|
UNIVERSUM UNIVERSITAS 20 September 2003 ALL RIGHTS RESERVED
This publication was prepared for personal use only.
|
|